Cyano substituted benzotriazole based polymers for use in organic solar cells
Casey, Abby; Green, Joshua P.; Tuladhar, Pabitra Shakya; Kirkus, Mindaugas; Han, Yang; Anthopoulos, Thomas D.; Heeney, Martin
JOURNAL OF MATERIALS CHEMISTRY A
2017
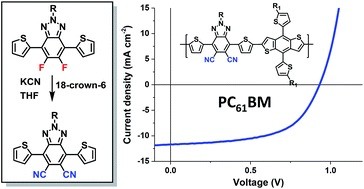
A new synthetic route to the electron accepting di-cyano substituted benzo[d][1,2,3]triazole (BTz) monomer 2-(2-butyloctyl)-4,7-di(thiophen-2-yl)-2H-benzotriazole-5,6-dicarbonitrile (dTdCNBTz) is reported. The cyano substituents can be easily introduced to the BTz unit in one step via the nucleophilic aromatic substitution of the fluorine substituents of the fluorinated precursor 2-(2-butyloctyl)-4,7-di(thiophen-2-yl)-2H-benzotriazole-5,6-difluoro (dTdFBTz). Co-polymers were prepared with distannylated benzo[1,2-b:4,5-b′]dithiophene (BDT) monomers containing either 2-ethylhexylthienyl (T-EH) side chains or 2-butyloctylthienyl (T-BO) side chains via Stille coupling to yield the novel medium band gap polymers P1 and P2 respectively. Whilst the organic photovoltaic (OPV) performance of P1 was limited by a lack of solubility, the improved solubility of P2 resulted in promising device efficiencies of up to 6.9% in blends with PC61BM, with high open circuit voltages of 0.95 V.