High-Performance Unipolar n-Type Conjugated Polymers Enabled by Highly Electron-Deficient Building Blocks Containing F and CN Groups
Zhang, Chan; Tan, Wen Liang; Liu, Zhongwei; He, Qiao; Li, Yanru; Ma, Jianeng; Chesman, Anthony S. R.; Han, Yang; McNeill, Christopher R.; Heeney, Martin; Fei, Zhuping
MACROMOLECULES
2022
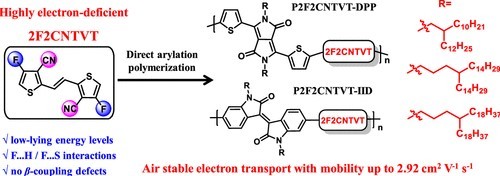
A highly electron-deficient building block, (E)-2,2′-(ethene-1,2-diyl)bis(4-chlorothiophene-3-carbonitrile) (2F2CNTVT), was designed and synthesized by the introduction of fluorine and cyano groups simultaneously onto the β position of thiophene units in (E)-1,2-di(thiophen-2-yl)ethene (TVT). A series of conjugated copolymers with different alkyl side chains were successfully synthesized via direct arylation polymerization (DArP) of 2F2CNTVT with thiophene-flanked diketopyrrolopyrrole (DPP) or isoindigo (IID) monomers. The resulting polymers exhibited improved planarity due to intramolecular or intermolecular F···S or F···H weak interactions and low-lying energy levels to realize stable and unipolar electron transport in organic thin film transistors (OTFTs). The microstructure and electrical performance of the polymers were confirmed to be dependent on the length of the side chains. Containing the longest side chains, P2F2CNTVT–DPP-3 and P2F2CNTVT–IID-3 displayed relatively higher μe of 2.03 and 2.92 cm2 V–1 s–1 with on/off ratios of up to 105–106, respectively, which was related to the favorable alignment of polymer chains for lower paracrystallinity and more ordered transport channels. Furthermore, the high-mobility OTFTs showed good air stability after a 1 month exposure to an ambient atmosphere due to their sufficiently low-lying LUMO levels. Our strategy of combining both F and CN groups in the same unit demonstrated its potential for high-performance n-type OTFT materials and can be extended to the design of new electron-deficient building blocks.