Influence of Backbone Fluorination in Regioregular Poly(3-alkyl-4-fluoro)thiophenes
Fei, Zhuping; Boufflet, Pierre; Wood, Sebastian; Wade, Jessica; Moriarty, John; Gann, Eliot; Ratcliff, Erin L.; McNeill, Christopher R.; Sirringhaus, Henning; Kim, Ji-Seon; Heeney, Martin
JOURNAL OF THE AMERICAN CHEMICAL SOCIETY
2015
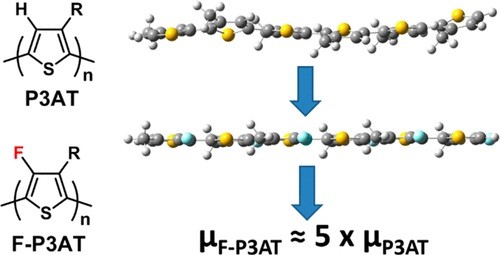
We report two strategies toward the synthesis of 3-alkyl-4-fluorothiophenes containing straight (hexyl and octyl) and branched (2-ethylhexyl) alkyl groups. We demonstrate that treatment of the dibrominated monomer with 1 equiv of alkyl Grignard reagent leads to the formation of a single regioisomer as a result of the pronounced directing effect of the fluorine group. Polymerization of the resulting species affords highly regioregular poly(3-alkyl-4-fluoro)thiophenes. Comparison of their properties to those of the analogous non-fluorinated polymers shows that backbone fluorination leads to an increase in the polymer ionization potential without a significant change in optical band gap. Fluorination also results in an enhanced tendency to aggregate in solution, which is ascribed to a more co-planar backbone on the basis of Raman and DFT calculations. Average charge carrier mobilities in field-effect transistors are found to increase by up to a factor of 5 for the fluorinated polymers.