Routes to some 3,6-disubstituted phthalonitriles and examples of phthalocyanines derived therefrom. An overview
Heeney, Martin J.; Al-Raqa, Shaya A.; Auger, Aurelien; Burnham, Paul M.; Cammidge, Andrew N.; Chambrier, Isabelle; Cook, Michael J.
JOURNAL OF PORPHYRINS AND PHTHALOCYANINES
2013
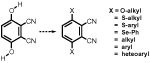
The paper reviews a selection of synthetic pathways that provide access to 3,6-disubstituted phthalonitriles, precursors for the synthesis of 1,4,8,11,15,18,22,25-octasubstituted phthalocyanine derivatives. Early routes using Diels–Alder reactions for the synthesis of 3,6-dialkyl, 3,6-dialkoxymethyl, 3,6-dialkenyl and 3,6-diphenylphthalonitriles are appraised. However, the emphasis of the review focuses on the scope and applications of 2,3-dicyanohydroquinone as a starting material for obtaining 3,6-disubstituted phthalonitriles. The earliest example of the use of 2,3-dicyanohydroquinone concerned its O-alkylation to afford 3,6-dialkoxyphthalonitriles. These are immediate precursors to near-infrared absorbing phthalocyanine derivatives. Triflation of 2,3-dicyanohydroquinone extends the scope of the compound for phthalocyanine synthesis; the bis-triflate derivative is susceptible to SNAr reactions and readily reacts with thiols to provide 3,6-bis(alkylsulfanyl) and 3,6-bis(arylsulfanyl)phthalonitriles. 3,6-Bis(phenylselenyl)phthalonitrile has also been obtained recently from the same precursor. Phthalocyanine derivatives obtained from them typically show a strongly bathochromically shifted Q-band absorption that is particularly sensitive to the central metal ion. The bis-triflate of 2,3-dicyanohydroquinone is also an ideal precursor for participation in cross-coupling reactions. Examples from the University of East Anglia group and elsewhere are presented which show the application of the nickel-catalyzed Negishi coupling reaction using alkylzinc halide derivatives. Yields of 3,6-dialkylphthalonitriles and 3,6-bis(substituted alkyl)phthalonitriles range from ca. 40 to 70%. Direct comparison for one example shows that the yield from the Negishi coupling method is higher than that using the Suzuki coupling protocol. Examples of the preparation of 3,6-diarylphthalonitriles from 2,3-dicyanohydroquinone bis-triflate using the Suzuki coupling reaction are reported with yields of the order of 65–70%. The review also includes a further application of 2,3-dicyanohydroquinone as a precursor to both monobromo and dibromo derivatives of 3,6-dibutoxyphthalonitrile. These compounds provide opportunities for cross-coupling at the brominated sites to provide more complex derivatives with the potential to serve as precursors of highly substituted phthalocyanine derivatives.